Preimplantation Genetic Testing for Aneuploidies (PGT-A)
What is PGT-A testing?
PGT-A is a powerful tool in modern fertility treatments, offering couples a higher chance of a healthy pregnancy and reducing the risks associated with chromosomal abnormalities.
PGT-A is a genetic test performed on cells taken from embryos produced through IVF. PGT-A can give information about the genetic health of your embryo to help your care team select an embryo for transfer, helping to improve your chances of achieving a successful pregnancy.
Chromosomes are structures made of protein and DNA that carry genetic information from the sperm and egg to the embryo. A chromosomally normal, or euploid, embryo contains 23 pairs of chromosomes amounting to 46 in total. One pair of chromosomes comes from the egg and the other the sperm. When an embryo doesn’t have the correct number of chromosomes it is called aneuploid. A euploid embryo is much more likely to result in successful implantation and the birth of a baby.
Advantages of PGT-A
- Increased Pregnancy Success Rates: By transferring chromosomally normal embryos, PGT-A improves implantation rates and reduces the likelihood of miscarriage.
- Reduced Risk of Miscarriage: Aneuploid embryos often fail to implant or result in early miscarriage. PGT-A helps identify such embryos, thereby reducing pregnancy loss.
- Supports Elective Single Embryo Transfer (SET): By selecting the most viable embryo, PGT-A lowers the risk of multiple pregnancies, which can pose health risks for both mother and babies.
- Tailored for Advanced Maternal Age: Women over 35 have a higher risk of aneuploid embryos. PGT-A helps optimize pregnancy outcomes for older women by ensuring that only euploid embryos (those with the correct number of chromosomes) are transferred.
- Reduced Need for Multiple IVF Cycles (Cost Effective): By identifying the best embryo(s) for transfer, PGT-A can potentially reduce the number of IVF cycles needed to achieve a successful pregnancy.
- Informed Family Planning: PGT-A offers couples the chance to make more informed decisions regarding their reproductive health, potentially reducing the emotional and financial toll of repeated IVF failures.
Who Can Benefit from PGT-A?
- Advanced Maternal Age: Women over 35 are more likely to produce embryos with chromosomal abnormalities, so PGT-A can be especially beneficial.
- Recurrent Miscarriages: Couples who have experienced multiple miscarriages may use PGT-A to reduce the likelihood of future pregnancy loss.
- IVF Failures: For couples with repeated IVF failures, PGT-A can help by identifying embryos with higher potential for successful implantation.
- Family Planning for Genetic Risks: Couples with a history of chromosomal abnormalities in previous pregnancies or within their family can benefit from this testing to avoid passing on these abnormalities.
As women age, the likelihood of producing chromosomally abnormal (aneuploid) embryos increases, while the number of normal (euploid) embryos decreases.
PGT-A can help patients of any age by improving their chances of a successful pregnancy. A large retrospective study showed that PGT-A significantly boosts live birth rates for patients across all age groups, with higher success rates compared to those who did not use PGT-A.
This underscores its value in enhancing pregnancy outcomes for all families.
Percentage of live births per embryo transferred increased across all age groups PGT versus non-PGT-A users.

1
Several cells are collected from your embryo while it remains in the care of your IVF center.
2
The cells from your embryo undergo analysis at our PGT-A testing lab.

3
A PGT-A report is sent to your clinician.
4
Based on the PGT-A report results, your clinician will help guide your transfer decision.
Preimplantation Genetic Testing for Monogenic Disorders (PGT-M)
PGT-M is an advanced reproductive technology designed to identify genetic abnormalities in embryos created through in vitro fertilization (IVF) before implantation.
This testing is crucial for couples who are carriers of specific inherited genetic conditions, allowing them to make informed decisions about their family planning and significantly reduce the risk of passing on serious genetic disorders to their children.
Conditions Commonly Screened with PGT-M
- Cystic Fibrosis
- Huntington’s Disease
- Sickle Cell Anemia
- Tay-Sachs Disease
- Muscular Dystrophy
- Thalassemia
Who is PGT-M for?
PGT-M is suitable for individuals at high risk of passing on a specific single-gene disorder. It may be considered in the following cases:
- Both partners are carriers of the same autosomal recessive condition (e.g., cystic fibrosis).
- One partner is a carrier of an X-linked condition (e.g., Duchenne muscular dystrophy).
- One or both partners have an autosomal dominant condition (e.g., Huntington’s disease).
- One or both partners carry a mutation linked to a hereditary cancer syndrome (e.g., BRCA1 & 2).
- You have had a child or pregnancy affected by a single-gene disorder.
- You want to perform HLA matching.
Benefits of PGT-M
- Disease Prevention: Significantly reduces the risk of having a child affected by a serious genetic disorder.
- Informed Decision-Making: Provides couples with critical information to make informed choices about their reproductive options.
- Increased Success Rates: Enhances the chances of a successful pregnancy by selecting the healthiest embryos for implantation.
- Emotional Relief: Offers peace of mind to parents who are carriers of genetic conditions, knowing they are taking proactive steps to ensure their child’s health.
Investing in PGT-M is a proactive step towards ensuring the health and well-being of your future children. Contact us today to learn more about how PGT-M can support your family’s genetic health and help you achieve your dreams of a healthy, genetically screened pregnancy.
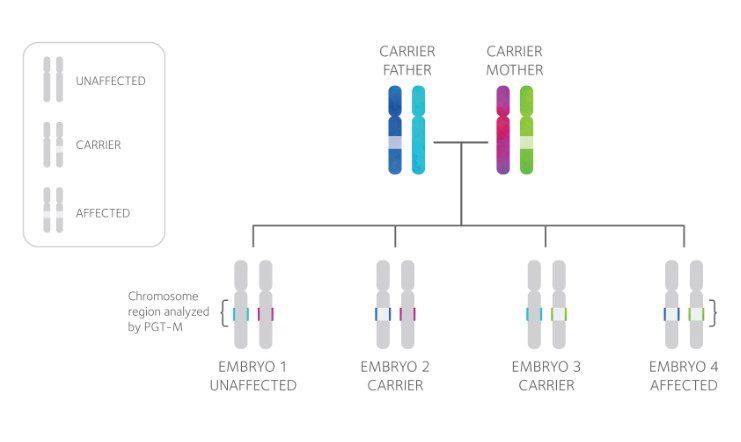
PGT-M technology
focuses on closely analyzing the specific genetic mutation carried by an individual, along with the surrounding chromosomal region, represented by the colored sections of the chromosomes above. Every PGT-M test is tailored to each family’s unique situation, requiring DNA samples from both partners and, in many cases, additional family members to develop the test. Linkage analysis is then used to establish the “genetic fingerprint” of the mutation, enabling a diagnosis of each tested embryo as either affected or unaffected.

Case review
Ordering provider submits a TRF along with genetic testing reports for case review and approval.

Genetic consultation
Registered patients speak with a genetic counselor (PGT-M: discuss if additional genetic testing is required).

PGT-M process
PGT lab collects DNA samples from the couple and family, and designs a test unique to each family.

IVF
In vitro fertilization is performed and the resulting embryos are incubated.

Embryo biopsy
An embryologist carefully removes a small cell sample from each embryo
Vitrification
Embryos are frozen while awaiting PGT results.
PGT-M
Biopsied samples are tested, and results are released to the IVF center.
Embryo transfer
A chromosomally normal embryo is selected for transfer or frozen for future use.

